Change of State
Change of State: Overview
This topic consists of various concepts like Change of State,Temperature Versus Time Graph for Heating of Ice,Fusion of Substances, etc.
Important Questions on Change of State
The latent heat of fusion of a substance is always less than the latent heat of vaporization or latent heat of sublimation of the same substance. Explain.
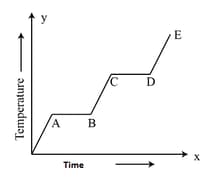
An ice cube is heated and the variation of its temperature with time is shown. The process representing the conversion of water into steam is
How much heat is required to convert of ice at to water at ? (Given specific heat of ice , specific heat of water , Latent heat of fusion )
of steam at is passed into a large block of ice at the mass of ice that melts is
Why does the cloud not getting frozen at very high height?
Write a short note on thermal equilibrium during change of state?
Define the boiling point of substance.
What is the thermal equilibrium?
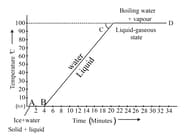
Identify the boiling point of water from the given temperature versus time graph for heating of ice.
What happens to a substance during fusion?
Identify the melting point of ice from the graph.
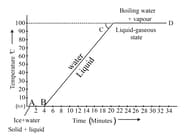
Explain latent heat of fusion of ice.
Define latent heat of fusion.
Water and ice having equal masses are placed inside an insulated container. The initial temperatures of water and ice are and respectively. The equilibrium temperature of the system in degrees Celsius is close to (Note: take the specific heat of water ; latent heat of fusion )
What does the principle of calorimetry state?
What is normal melting point?
The physical quantity that determines whether or not a given system is in thermal equilibrium with another system is called
